Primary endpoint data
In STELLAR, a 24-week study of adult patients with PAH on stable background therapy1:
WINREVAIR significantly increased exercise capacity, as measured by an improvement in 6MWD from baseline
PRIMARY ENDPOINT
Placebo-adjusted median increase in 6MWD at week 24
Placebo-adjusted change from baseline in 6MWD (meters) at week 24 in subgroups
aHodges-Lehmann location shift from placebo estimate (median of all paired differences).
Change from baseline in 6MWD at week 24 for subjects who died was imputed to -2000 meters to receive the worst rank. Change from baseline in 6MWD at week 24 for subjects who had missing data due to a non-fatal clinical worsening event was imputed to -1000 meters to receive the next-worst rank.
ASE = asymptotic standard error; CHD = congenital heart disease; CI = confidence interval; s/p = systemic-to-pulmonary.
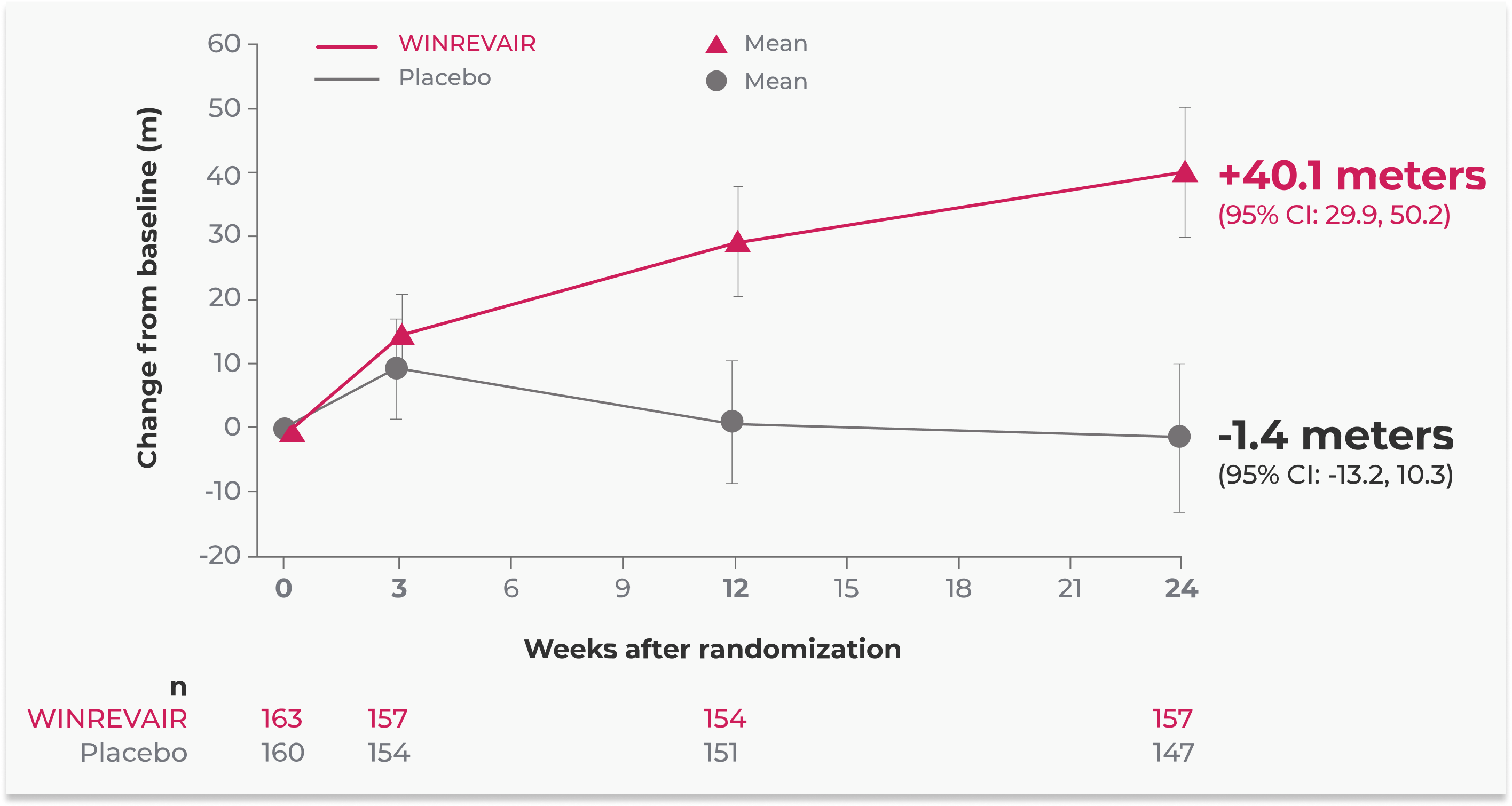
Additional Analysis: Mean change (observed) in 6MWD through week 241
The primary endpoint in the STELLAR trial was the change from baseline in 6MWD at 24 weeks. The primary analysis reported in the Prescribing Information for WINREVAIR is the placebo-adjusted median increase in 6MWD (in meters) This graph shows the observed mean changes in 6MWD (in meters). Walking distance was recorded at prespecified trial visits (weeks 0, 3, 12, and 24) during the first 24 weeks of the trial. The data shown are for patients with available data (observed) over time.
The confidence intervals (indicated by I bars) have not been adjusted for multiplicity and cannot be used to infer definitive treatment effects.
Reference:
- Hoeper MM, Badesch DB, Ghofrani HA, et al; STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490.
