Recommended dosage and injection volume calculator for WINREVAIR (sotatercept-csrk)
WINREVAIR is administered once every 3 weeks by subcutaneous injection, according to patient body weight
The recommended STARTING DOSE is 0.3 mg/kg
- Obtain hemoglobin (Hgb) and platelet count prior to the first dose of WINREVAIR.
- Do not initiate treatment if platelet count is <50,000/mm3 (<50×109/L).
Injection volume for starting dose is calculated based on patient weight as follows:


Scroll down to view additional dosage information and injection volume calculator.
Injection volume should be rounded to the nearest 0.1 mL.
For example: (70 kg x 0.3 mg/kg) ÷ 50 mg/mL = 0.42 mL, rounds to 0.4 mL.
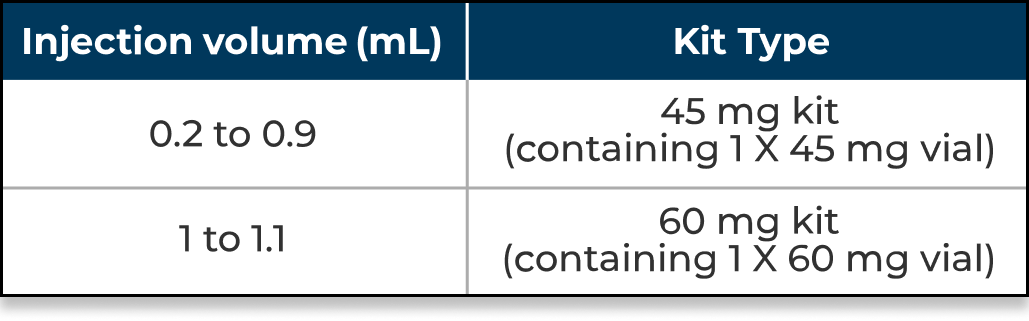
See the table below for selecting the appropriate kit based on calculated injection volume for starting dose.
Kit type based on injection volume for dose of 0.3 mg/kg

The recommended TARGET DOSE is 0.7 mg/kg
- After verifying acceptable Hgb and platelet count, increase to the target dose of 0.7 mg/kg.
- Continue treatment at 0.7 mg/kg every 3 weeks unless dosage adjustments are required.
Injection volume for target dose is calculated based on patient weight as follows:

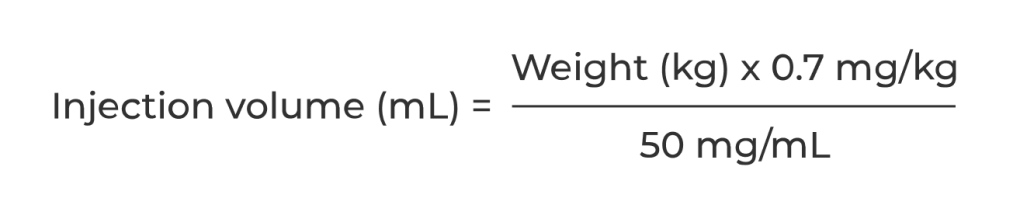
Injection volume should be rounded to the nearest 0.1 mL.
For example: (70 kg x 0.7 mg/kg) ÷ 50 mg/mL = 0.98 mL, rounds to 1 mL.
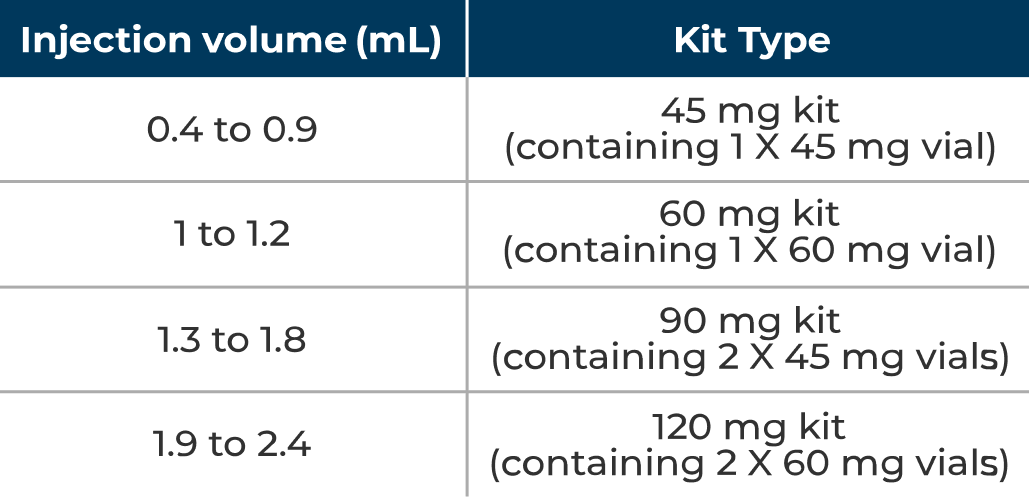
See the table below for selecting the appropriate kit based on calculated injection volume for target dose.
Kit type based on injection volume for dose of 0.7 mg/kg

Missed dose, overdose, and underdose
- If a dose of WINREVAIR is missed, administer as soon as possible.
- If the missed dose of WINREVAIR is not administered within 3 days of the scheduled date, adjust the schedule to maintain 3-week dosing intervals.
- In case of an overdose, monitor for erythrocytosis.
Dosage modifications due to Hgb increase or platelet count decrease

Check Hgb and platelet count before each dose for the first 5 doses, or longer if values are unstable. Thereafter, monitor Hgb and platelet count periodically.
Click button below to access the injection volume calculator


Delay treatment for at least 3 weeks if any of the following occur:
- Hgb increases >2.0 g/dL from the previous dose and is above upper limit of normal (ULN)
- Hgb increases >4.0 g/dL from baseline
- Hgb increases >2.0 g/dL above ULN
- Platelet count decreases to <50,000/mm3 (<50.0 x 109/L)
Recheck Hgb and platelet count before reinitiating treatment. For treatment delays lasting >9 weeks, restart treatment at 0.3 mg/kg and escalate to 0.7 mg/kg after verifying acceptable Hgb and platelet count.
